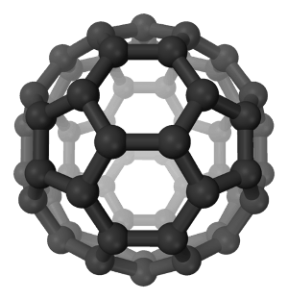
What is Carbon 60?
Carbon 60 is a tiny molecule composed of 60 carbon atoms arranged in a sphere, and is also known as a buckyball.
Buckyballs are members of the fullerene family of carbon structures, which also include spheres, tubes, ellipsoids and a variety of other shapes.
Fullerenes can range from 20 carbon atoms up to as many as 100 carbon atoms, and are of great interest to researchers for both their chemical properties and possible applications in industry and technology.
Where does Carbon 60 come from?
Carbon 60 fullerene was first discovered and manufactured in 1985 by four scientists, who named it after Buckminster Fuller, an American architect and inventor who popularised the geodesic dome, and which look similar to spherical carbon 60 molecules.
Some forms of fullerene, including C60, C72, C76, C82 and C84 have been found to occur naturally, in soot, lightning discharges and also in the mineral shungite. They’ve also been found in the dust around stars, suggesting that buckyballs have existed for a very long time in nature.
Most carbon 60 is manufactured in the laboratory, using an electric discharge between carbon electrodes to create a soot from which the carbon 60 fullerene molecules can be extracted.
What’s interesting about Carbon 60?
Carbon 60 fullerene and other buckyballs have a number of properties that make them very interesting to researchers and industry.
They are relatively stable molecules, and are not easily dissolved in water or other solvents. Their spherical shape can trap other atoms inside them, they can act as super conductors, and they have been shown to behave as both a wave and a particle according to quantum physics.
They can also withstand high temperatures and pressures, and the carbon 60 molecules can react with other atoms while preserving their spherical shape. Carbon 60 can be oxidised, happily taking on extra electrons, although this can be reversed.
Individual molecules of carbon 60 fullerene transmit either blue or red light, which creates a purple colour for pure preparations of Carbon 60.
Solid carbon 60 is soft, like the graphite in lead pencils, but turns into a superhard form of diamond under extreme pressure. It also absorbs light at a significantly higher rates, when it is subjected to higher-intensity light.
And research in 2012 on the toxicity of Carbon 60 showed that not only was it not toxic at all to rats, but that it almost doubled their lifespan, possibly due to the fact that it is a highly effective antioxidant, with the ability to “mop up” free radicals hundreds of times better than standard anti-oxidants.
What is Carbon 60 used for?
The way that carbon 60 absorbs light and attracts electrons makes it an excellent substance to use in solar cells, for harvesting energy from the sun.
Buckyballs have also been used for lubrication, and show promise for use as electrical conductors and superconductors.
Other research suggests that fullerene buckyballs might be used to store hydrogen, as a fuel source for hydrogen-powered cars, and certain manufacturers have created non-carbon fullerene structures for use in bulletproof vests.
Carbon 60 buckyballs might also be able to used to reduce bacterial growth in water systems.
In the biomedical industry, researchers are exploring whether buckyballs can:
- reduce inflammation from allergic reactions
- fight the degeneration that occurs during multiple sclerosis
- bind to the proteins in HIV to prevent its spread
- reduce aging and extend the lifespan of mammals, including humans
Further reading
- http://www.ch.ic.ac.uk/rzepa/mim/century/html/c60_text.htm
- https://en.wikipedia.org/wiki/Fullerene
- https://en.wikipedia.org/wiki/Buckminsterfullerene
- https://www.scientificamerican.com/article/have-buckminsterfullerene/
- http://www.understandingnano.com/buckyballs-fullerenes.html
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4124742/